**InsightAce Analytic Pvt. Ltd. Announces Release of Market Assessment Report on Global Rare Disease Clinical Trials Market**
InsightAce Analytic Pvt. Ltd. has announced the release of a comprehensive market assessment report titled **“Global Rare Disease Clinical Trials Market Size, Share & Trends Analysis Report By Therapeutic Area (Oncology, Cardiovascular Disorders, Neurological Disorders, Infectious Disease, Genetic Disorders, Autoimmune and Inflammation, Hematologic Disorders, Musculoskeletal Disorders), Phase (Phase I, II, III, and IV), And Sponsor (Pharmaceutical & Biopharmaceutical Companies, Non-profit Organizations) – Market Outlook And Industry Analysis 2031.”**
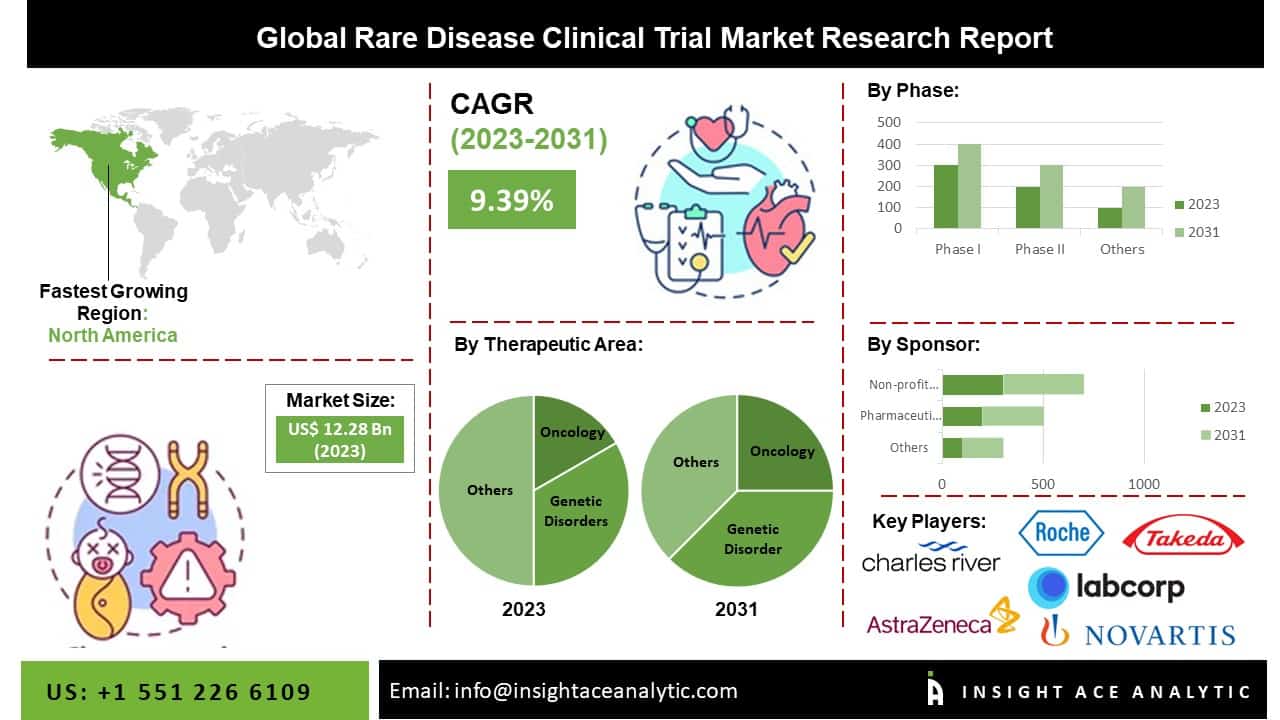
The global Rare Disease Clinical Trials market is estimated to reach over **USD 25.05 billion by 2031**, exhibiting a strong CAGR of **9.39%** during the forecast period.
—
### Impact of COVID-19 on Rare Disease Clinical Trials
The COVID-19 pandemic led to a global suspension of elective treatments, including diagnostic and therapeutic procedures, significantly impacting the rare disease treatment market. A review published in *Frontiers in Public Health* in April 2021 highlighted the challenges faced by individuals with rare disorders during this period, noting that regular clinical care, counseling, and therapies were insufficient.
Despite these challenges, several factors are driving ongoing growth in the market. These include increased research and development activities for novel therapeutics and drugs, a rise in the number of new drug launches, growing prevalence of rare diseases, supportive vaccination initiatives, and favorable government policies.
—
### Prominent Players in the Rare Disease Clinical Trials Market
– Takeda Pharmaceutical Company
– Hoffmann-La Roche Ltd.
– Pfizer, Inc.
– Charles River Laboratories
– Icon PLC
– Parexel International Corporation
—
### Market Dynamics
#### Drivers
The rare disease treatments market is expanding due to multiple key factors:
– Advancements in treatment innovations
– Increasing product approvals
– Strategic collaborations and acquisitions by major pharmaceutical companies
For example, in May 2022, the European Medicines Agency (EMA) approved **Xenpozyme (olipudase alfa)** for the treatment of non-central nervous system (CNS) aspects of Acid Sphingomyelinase Deficiency (ASMD), a rare and progressive genetic disorder.
#### Challenges
A significant challenge in conducting clinical trials for rare diseases is the necessity of multicenter, often international, studies. This is critical to ensure sufficient patient recruitment, especially during phases I and II. However, it complicates the coordination of procedures, ethical reviews, indemnity agreements, clinical service organization, and management of cultural diversity.
—
### Regional Trends
**North America** is expected to maintain its dominant share of the global rare disease treatments market. This is driven by the high prevalence of rare diseases such as Huntington’s disease, spina bifida, fragile X syndrome, Guillain-Barré syndrome, Crohn’s disease, cystic fibrosis, and Duchenne muscular dystrophy.
The region benefits from heightened awareness of rare diseases and a robust healthcare system well-equipped to diagnose and treat such conditions. According to the Genetic and Rare Diseases (GARD) Information Center:
– Approximately **7,000 rare diseases** have been identified
– One in ten Americans (about **30 million people**) are affected by a rare disease
Similarly, the Canadian Organization for Rare Disorders (CORD) reported in 2021 that one in twelve Canadians is impacted by a rare disease annually, with genetic mutations responsible for 80% of these cases.
As such, North America is poised for substantial growth in this market in the coming years.
—
### Recent Developments
– **January 2023:** Genethon, an R&D company, initiated a crucial clinical trial involving gene therapy for treating **Crigler-Najjar Syndrome**, a rare genetic liver disease characterized by abnormally elevated bilirubin levels (hyperbilirubinemia).
– **November 2022:** Protalix Biotherapeutics Inc. and Chiesi Global Rare Diseases re-submitted the Biologics License Application (BLA) for **PRX-102 (pegunigalsidase alfa)** to the US Food and Drug Administration (FDA), targeting adult patients with Fabry disease.
—
### Market Segmentation
The Rare Disease Clinical Trials market is segmented as follows:
**By Therapeutic Area:**
– Oncology
– Cardiovascular Disorders
– Neurological Disorders
– Infectious Disease
– Genetic Disorders
– Autoimmune and Inflammation
– Hematologic Disorders
– Musculoskeletal Disorders
– Others
**By Phase:**
– Phase I
– Phase II
– Phase III
– Phase IV
**By Sponsor:**
– Pharmaceutical & Biopharmaceutical Companies
– Non-profit Organizations
– Others
**By Region:**
– **North America:** The US, Canada, Mexico
– **Europe:** Germany, The UK, France, Italy, Spain, Rest of Europe
– **Asia-Pacific:** China, Japan, India, South Korea, Southeast Asia, Rest of Asia Pacific
– **Latin America:** Brazil, Argentina, Rest of Latin America
– **Middle East & Africa:** GCC Countries, South Africa, Rest of Middle East and Africa
—
### Request a Free Sample
To get free sample pages or request specific information from the report, please contact InsightAce Analytic Pvt. Ltd.
—
### About InsightAce Analytic Pvt. Ltd.
InsightAce Analytic is a market research and consulting firm that empowers clients to make strategic decisions. The firm provides both qualitative and quantitative market intelligence solutions to identify untapped markets, explore new and competing technologies, segment potential markets, and reposition products.
With expertise in delivering syndicated and custom market intelligence reports, InsightAce offers in-depth analysis and key market insights in a timely and cost-effective manner.
—
### Contact Information
– Email: [email protected]
– Website: [www.insightaceanalytic.com](http://www.insightaceanalytic.com)
– Tel (USA): +1 607 400-7072
– Tel (Asia): +91 79 72967118
Follow us on Twitter: [@InsightAce](https://twitter.com/InsightAce)
—
Stay informed with InsightAce Analytic for the latest insights and comprehensive analysis of the Rare Disease Clinical Trials market.
https://www.prnewsreleaser.com/news/115921